Diversified biopharmaceutical company CLINUVEL’s (ASX:CUV) drug afamelanotide has been administered to the first patient diagnosed with an acute arterial ischaemic stroke (AIS) enrolled in a world’s first clinical trial (CUV801).
AIS accounts for approximately 85% of the 15 million strokes suffered worldwide each year. Despite its prevalence, treatment options are limited. In Europe, over 85% of AIS cases presenting to hospitals are not eligible for current standard of care treatment (thrombectomy and thrombolysis).
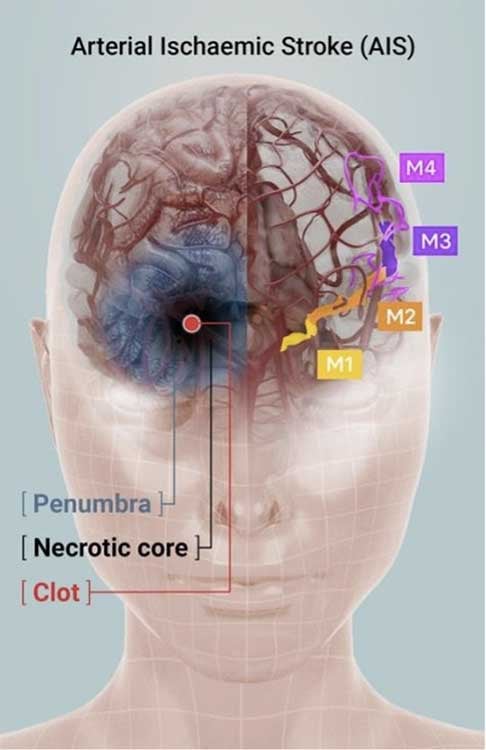
Due to a clot formed in the higher region of the left middle cerebral artery (M2 or above), the patient suffered an acute stroke and was admitted to a specialist neurological hospital in Australia to receive treatment.
CLINUVEL’s Clinical Operations Manager, Dr Pilar Bilbao, said in total, six adult AIS patients will be evaluated in the Phase II CUV801 study. The study focuses on the safety and therapeutic potential of afamelanotide in patients who are ineligible for standard stroke therapy.
“The immediate aim in acute AIS treatment, is to bring back the patient’s neurological and muscular functions by improving the blood flow to the affected site of the brain,” Dr Bilbao said. “Our unambiguous aim is to develop a treatment for 70% to 80% of the stroke patients who currently have no alternative treatment”.
Scientific progress has demonstrated melanocortins, including afamelanotide, provide a positive effect on the central nervous system (CNS). Afamelanotide is known to offer neuroprotection and act as a potent anti- oxidative hormone. The drug possesses further therapeutic benefits, activating vessels, reducing fluid formation, protecting critical nerve and brain tissue, and restoring the blood brain barrier (BBB: a critical defence mechanism protecting the brain).
The drug therapy is expected to affect the blood flow and oxygen to deprived brain tissue.
Stroke is the second most common reason of death and a leading cause of disability worldwide, yet many stroke patients are unsuitable to receive the current standard of care (clot removal and clot dissolution).
Acute stroke most frequently occurs unexpectedly and without warning. The main reason for AIS is a severe constriction of, and a clot lodged within, the brain vessel. AIS leads to an immediate lack of oxygen and glucose supply, resulting in partial death of brain tissue. A stroke patient may suddenly lose consciousness, and typically will experience loss of movement of one side of the body (such as the arms, legs or face).
Following a stroke, family and bystanders usually ensure that the patient is immediately transported to the emergency department of a hospital, where a brain scan (computed tomographic angiography; CTA) and clinical examination need to confirm the diagnosis. Partial or full recovery of the patient depends on the size of the infarct (dead brain tissue) incurred, speed of treatment offered, and underlying general health.
Unfortunately, the majority of stroke patients do not receive standard therapy because it cannot be offered within the internationally accepted critical treatment window of four and a half hours, or because the clot is lodged in inaccessible parts of the brain (M2 branch and higher).
CUV801 STUDY – AFAMELANOTIDE IN AIS
The CUV801 study is evaluating the use of afamelanotide in six patients who suffered an acute stroke, with a main focus on safety monitoring following drug administration as they are admitted to hospital.
Following multiple dosing, patients enrolled in the study are clinically assessed to detect changes or improvement in neurological functions and activities of daily living. Validated clinical tools, the Modified Rankin Scale and National Institutes of Health Stroke Scale, are used to evaluate the extent of patients’ disability.
Through a number of periodic magnetic resonance imaging (MRI) brain scans, the blood volume and flow to the affected regions of the brain are assessed, with a special attention to the core of the stroke (infarct) and penumbra.




