AdAlta Limited (ASX:1AD) has obtained new data supporting the potential efficacy of AD-214 in human patients with Idiopathic Pulmonary Fibrosis (IPF) and other fibrotic diseases, when administered using clinically feasible dosing regimens.
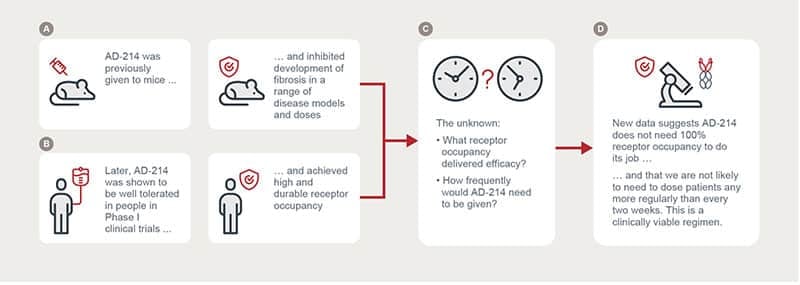
This new data is critically important. We have known AD-214 is efficacious in animal models of fibrotic diseases and also that it can at least partially block its target receptor for several days and even weeks after a single IV infusion. What we have not known is whether we could replicate the therapeutic effect seen in animals at these levels of receptor occupancy and hence at dosing frequencies acceptable in humans,” AdAlta CEO and Managing Director, Dr Tim Oldham, said.
“For the first time we have been able to show that we can maximally inhibit a key fibrotic process with as little as 60% receptor occupancy and that meaningful inhibition can be achieved at much lower levels.
“This supports the hypothesis that AD-214 may be able to be dosed at a clinically convenient frequency of no more than every two weeks. This information is also extremely valuable for determining appropriate dosing for AD-214 Phase II clinical studies.
“Linking a clinically measurable parameter (receptor occupancy) with efficacy answers a question commonly asked by potential commercial partners and substantially reduces the risk of Phase II studies. The completion of these difficult experiments is a credit to our in-house scientific team.”
Study overview
AdAlta is developing AD-214 as a first-in-class treatment for fibrotic diseases such as IPF. AD-214 targets and blocks the chemokine receptor CXCR4 that is a receptor involved in several cellular processes involved in fibrosis, including the migration of immune, inflammatory and fibrotic cells to the sites of injury and disease.
In earlier preclinical studies, AD-214’s efficacy was demonstrated in animal models of lung, kidney and eye fibrosis, and AD-214 was found to inhibit several fibrotic processes. AD-214 was also shown to be safe and well tolerated in healthy volunteers in a Phase I clinical study. During that Phase I study, it was also observed that AD-214 achieved more than 50% blocking (or receptor occupancy) for at least a week at 5 and 10 mg/kg and up to three weeks at 20 mg/kg.
Clinical convenience requires at least two weeks between IV infusions. The optimal level of sustained receptor occupancy required for therapeutic effect was yet to be determined.
The new ex vivo studies simultaneously measured AD-214’s ability to occupy CXCR4 receptors and to inhibit a key fibrotic process, the migration of cells expressing CXCR4 on their surface. AD-214 concentrations included those low concentrations observed in blood several days after IV infusion in Phase I clinical studies. The studies were able to replicate the CXCR4 receptor occupancy observed in Phase I clinical studies at these low concentrations. Significantly, even when receptor occupancy was substantially less than 100%, material inhibition of cell migration was observed.
The new data is the first time a clinically measurable parameter (receptor occupancy) has been linked to an antifibrotic mechanism (cell migration) and supports the hypothesis that intravenous administration of AD-214 every two weeks or more could be efficacious.
The results of the upcoming Phase I extension study and detailed pharmacokinetic simulation studies will now be able to optimise dosage and dose scheduling for Phase II studies with increased confidence, substantially reducing the risks associated with conducting those Phase II studies.




