Brisbane based AnteoTech Ltd (ASX: ADO) has been awarded up to $1.4 million towards the further development and commercialisation of its COVID-19 Antigen Rapid Test (ART), under the Queensland Government’s Essential Goods and Supply Chain Program (EGSCP). AnteoTech will draw down up to $1.4 million against agreed milestones.
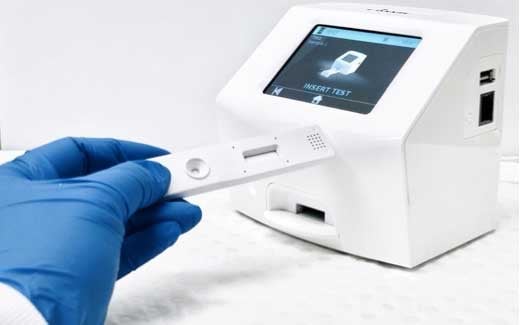
The EGSCP programme aims to help Queensland manufacturers make essential goods such as personal protective equipment, health consumables and devices, particularly in response to the COVID-19 pandemic. AnteoTech’s submission focused on the development and commercialisation of its proposed 15-minute point of care COVID-19 Antigen Rapid Test (ART), harnessing AnteoTech’s nanoparticle surface management technology AnteoBind and a Europium particle to provide high sensitivity fluorescent detection of the SARS-CoV-2antigen.
CEO, Derek Thomson said development of the product to this point has included presentations and assessment of the COVID-19 ART development by senior members of Queensland Health and Queensland Pathology.
The grant term is two years, with drawdowns awarded upon achievement of commercialisation objectives including the development of working test prototypes, reader platform completion, TGA approval, manufacture of 1 million tests per annum in Queensland and associated employment of a number of additional staff.
Mr Thomson said that over the past 12 months, AnteoTech has increased its capabilities in Assay Development and achieved ISO-13485 certification (certification number MD 723665) to become a legal manufacturer of medical devices.
We are delighted to be awarded these funds under the Essential Goods and Supply Chain Program. The award of the grant is a strong endorsement of AnteoTech’s development and commercialisation progress. It’s a testament to the great work that Dr Charlie Huang, our Head of Life Sciences and his team have undertaken over the past nine months in developing the test,” Mr Thomson said.
“As we move through clinical trials and regulatory approval of our ART, we are excited to be part of the growing Queensland response to the COVID-19 pandemic. We have observed significant investment and ramping up of manufacturing capabilities right here in South East Queensland and are looking forward to entering into commercial contracts with local businesses for the manufacturing of our test once regulatory approvals have been obtained. On product launch, we will have built capacity to produce a large volume of tests at our facility at Eight Mile Plains.
“Recognition by the Qld Government through awarding the grant is an excellent step in the development of our COVID-19 Antigen Rapid Test. We look forward to further engagements with interested companies and agencies, including QLD Health and building a strong relationship through the collaboration on trials and further development of the test.”




